600,000 Bottles of Blood Pressure Medication Recalled Due to Contamination Concerns
Over 600,000 bottles of a popular blood pressure medication, Ramipril, have been recalled in the United States. Concerns rose after discovering the drug might be contaminated because key ingredients were sourced from an unapproved Indian factory.
Ramipril is a widely prescribed medication, given to over 2.4 million Americans annually to help lower blood pressure. The medication works by causing the blood vessels to widen, thus lowering blood pressure.
According to the Food and Drug Administration (FDA), the risk posed to the public from this batch of Ramipril is considered low. Importantly, there have been no reported adverse reactions or health events associated with its use so far.
The Recall Details
The recall concerns capsules produced by Lupin Pharmaceuticals, an Indian company. These capsules are available in dosages of 2.5 mg, 5 mg, and 10 mg, packaged in bottles containing either 90, 100, or 500 pills. All affected batches have a best before date stretching up to July 2026.
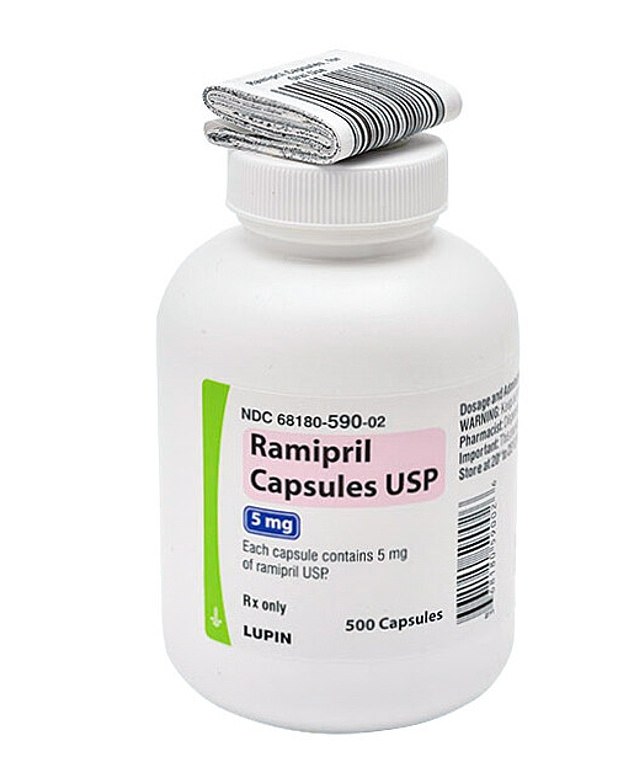
The manufacturing took place in Goa, India. Initially, the recall was announced on October 23, affecting the 10 mg and 5 mg variants. By November 19, it expanded to cover the 2.5 mg strength as well.
During an FDA inspection, it was discovered that a crucial ingredient in the medication, known as the Active Pharmaceutical Ingredient (API), originated from a different manufacturer that lacked agency approval.
Advice for Patients
If you happen to own any of these recalled bottles, the FDA advises you to either discard them or return them to where they were purchased for a full refund. It is wise to contact your healthcare provider to discuss alternative treatments or get a new prescription.

The manufacturer’s name, Lupin Pharmaceuticals, is also available on the FDA’s website detailing all the specific recalled batches. For obvious reasons, finer details are crucial for sorting any complications effectively.
Broader Context of Drug Recalls
This recall comes amidst several other pharmaceutical recalls in recent times, particularly from Indian manufacturers.
Just a few weeks earlier, Dr. Reddy’s Laboratories, an Indian pharmaceutical company, recalled more than 330,000 bottles of tablets contaminated with a carcinogenic impurity called nitrosamine. These tablets were primarily used to treat hyperparathyroidism—a condition impacting thousands of patients every year by elevating calcium levels in the blood.
Moreover, last year saw a massive recall of eye drops from India after they were linked to bacterial contamination. This contamination led to several cases of serious infections and even fatalities due to severe sepsis.
Understanding Recall Classes
Now, talking about recalls, it’s vital to understand what a Class II recall means. Generally, this classification implies that the product has a low probability of causing serious health consequences or death.
A Class I recall represents a more severe situation where there is a reasonable chance of severe health consequences or fatality.
For peace of mind, consumers should always remain vigilant about the medications they take. Whether it involves consulting healthcare providers or staying informed about official FDA recalls, such steps ensure your safety and well-being remain uncompromised.
In conclusion, while the case with Ramipril is a good reminder of the complexities surrounding pharmaceutical manufacturing and safety, the FDA continues taking measures to ensure public health is safeguarded, reassuring us all in the process.





